Diferenciación de Células T
Introducción
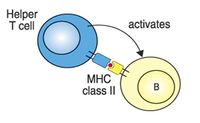
Las células T son de larga vida y están involucrados en la inmunidad mediada por células. Funcionalmente se dividen por la expresión de marcadores CD4+ o CD8+. Las células T CD4+ cooperadores reconocen los antígenos fijados a complejos MHC II y están involucradas en el control de patógenos intracelulares y extracelulares, ya que pueden interactuar con CD8+, NK y células dendríticas o células B. Las células T CD8+ citotóxicas reconocen el complejo MHC I y destruiyen las células infectadas o neoplásicas.
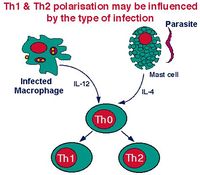
Dentro de la sangre y los órganos linfoides la mayoría de las células T son inespecíficas a para cualquier antígeno, y sólo hay una pequeña proporción de células T memoria. Las células T vírgenes aún tienen que encontrar un antígeno, el cual debe ser presentado por las células dendríticas. Después de la activación antigénica inicial, células T inespecíficas se convierten en una célula de etapa intermedia llamada TH0, que puede ser activada por cualquier célula presentadora de antígeno, por ejemplo, las células dendríticas, macrófagos o células B.
Las células TH0 tienen la capacidad de diferenciarse en TH1 y TH2. El tipo de célula en que se desarrolla depende del tipo de antígeno que sea presentado. La IL-12 producida por macrófagos favorece el desarrollo de TH0 a TH1. Las células B causan la transformación hacia TH2inducida por IL-10. Tras la estimulación antigénica de TH1 o TH2, se produce la expansión clonal y se secretan una amplia gama de diferentes citoquinas.
Este estado de secreción es de corta duración (de 4 a 40 horas). Después de este tiempo estas células mueren o maduran en células de memoria de larga duración. La proliferación de las Células T continúa hasta que la presentación del antígeno cesa.
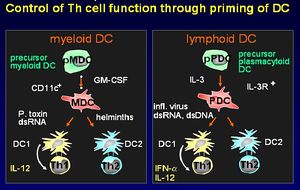
Las Células Dendríticas
Hay dos linajes diferentes de células dendríticas:
- Precursoras de células mieloides.
- Precursoras de células plasmacitoides.
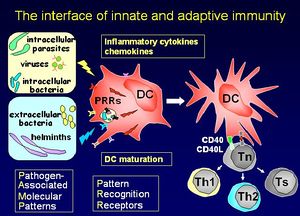
Las células dendríticas estimulan una respuesta por parte de células T primarias. Migran a través de los tejidos realizando un seguimiento a las células T hasta las áreas de los ganglios linfáticos. Las células dendríticas tienen una capacidad única para tomar antígenos por medio de fagocitosis, macropinocitosis y pozos recubiertos de clatrina. El fenotipo de superficie distingue a las células dendríticas de Monocitos/macrófagos y células B. Su función principal es preparar a las células T cooperadoras. Producen moléculas de señalización de células conocidas como quimiocinas.
Señales de Maduración
- Exógenas:
- Las bacterias o sus productos (LPS, LTA, lipoproteínas).
- Virus o sus productos (dsRNA, G-RSV).
- Protozoos o sus productos.
- Helmintos (SEA, ES 62).
- Endógenos
- Mediadores de la inflamación (IL-1/TNF-a, HSP, FCR).
- Las células inmunes (CD40L, CD47, FasL).
Presentación de Antígenos
Los monocitos circulantes se diferencian para formar células dendríticas inmaduras llamadas células de Langerhans
Las células de Langerhans muestrean el líquido circundante a los tejidos por endocitosis con objeto de:
- Buscar organismos ajenos que internalizan.
- Dentro de las células dendríticas, el antígeno ingerido es digerido hasta que sólo quedan sus péptidos.
- Algunos de los péptidos formados se unen a las moléculas MHC.
Las células de Langerhans abandonan el epitelio y viajan a través del flujo de linfa aferente. A partir de entonces se conocen como células veliformes. Las células veliformes entran en la región paracortical de los ganglios linfáticos, donde presentan antígenos a las células T. Es ahora cuando se conocen como células dendríticas interdigitales.
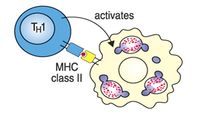
Células TH1
Las células TH1 ayudan a los macrófagos a que digieran bacterias.
Algunas de las citoquinas secretadas por las células TH1 incluyen:
- IL-2, which induces proliferation of both CD4+ and CD8+ T-cells. This stimulation of T cell proliferation is the main function of the TH1 cell.
- Interferon gamma (IFNγ) which activates tissue macrophages and is the principal effector mechanism in the defence against intracellular bacteria and parasites such as Mycobacteria, Brucella, Rickettsia Leishmania, Coccidia, and Babesia. IFNγ activates macrophages and stimulates them to produce enzymes triggering intracellular killing mechanisms - specifically:
- Superoxide dismutase and myeloperoxidase that produce H2O2 and trigger the "superoxide burst".
- Nitric oxide synthase which produces nitric oxide.
This is another example of the immune system working through the innate immune response - this can act to suppress antibody synthesis.
TH2 Cells
TH2 cells help B cells produce antibody where the organism in present in tissue fluid. The TH2 population influences B cell activation, proliferation and immunoglobulin production. TH2 T cells also secrete a range of cytokines:
- IL-4 which stimulates B cell growth and induces the heavy chain switch from IgM to IgG , IgA and IgE, as well as proliferation of basophils and mast cells. IL-4 can inhibit some T cell responses.
- IL-5 which activates B cells and stimulates a high rate of proliferation. IL-5 also promotes immunoglobulin synthesis and the proliferation and differentiation of eosinophils.
- IL-6 also activates B cells, stimulates a high rate of proliferation and promotes immunoglobulin synthesis.
Common Functions of Th1 and TH2 Cells
Both TH1 and TH2 cells produce IL-3 and granulocyte-macrophage colony stimulating factor (GM-CSF). These act to activate and induce proliferation of neutrophils and macrophages. Neutrophils are the major phagocytic cells in the blood and the principal cells in acute inflammatory lesions whose function is chiefly the body's defence against extracellular bacteria. One of the major biological functions therefore of the activation of either TH subset is cytokine-controlled reactive haematopoiesis.
Cytotoxic T-Cells
Cytotoxic T cells kill virus infected cells where the organisms are contained in the cell cytoplasm. Viruses are obligate intracellular pathogens that use the host cell machinery for pathogen protein synthesis; viral peptides associate with MHC class I and are expressed on the cell surface. CD8+ cytotoxic T lymphocytes (CTL) recognise the antigen-MHC complex. Cytotoxic T-cells secrete a pattern of cytokines similar to that of TH1 cells:
- IFNγ but not IL-2. The IFNγ shifts the balance of the immune response in favour of TH1 cells and there is therefore an increased level of T-cell proliferation. The initiation of the immune response via CTL leads to the selective proliferation of CTL which enhances the main mechanism of killing virally-infected cells.
Killing Mechanism
The CTl killing mechanism is initiated by direct CTL-target cell contact.
- The cells involved bind by antigen/MHC class I-TcR interaction. This allows the CTL's intracellular granules to be localised at the area of contact - the granules contain most of the molecules responsible for cytotoxicity.
- Direct cell contact stimulates the release of the granule contents into the area of contact between the two cells. The granules contain two groups of cytotoxic molecules.
- Perforin, which is structurally related to the complement component, C9 and forms pores in the cell membrane.
- Granzymes, which are proteolytic enzymes that target cell nucleases and cause programmed cell death.
T-Cell Activation
T cells function only after recent activation by an antigen.
- CD4 binds MHC class II - CD4+ T-cells recognise antigen only in association with MHC class II.
- CD8 binds MHC class I - CD8+ T-cells recognise antigen only in association with MHC class I.
Activation of T cells requires two distinct signals:
- Signal 1 is the interaction of the TcR with the antigenic peptide/MHC complex on the antigen presenting cell.
- Signal 2 is the interaction of CD28 on the T cells with its ligand, CD80, on the antigen-presenting cell (APC). APC expression of CD80 only occurs after the engagement of pattern recognition on Fc receptors or activation by the cytokines Interferon, IL-1β or TNFα.
Signal 2 only occurs after the recognition of DANGER.
Activation Scenarios
1. No signal 1:
- T cell is not activated as there is no antigen.
2. Both signal 1 and signal 2
- T cell is activated into clonal expansion and produces cytokines or becomes cytotoxic.
3. Signal 1 but no signal 2
- T-cell is triggered into apoptosis and dies.
- This is the basis of "clonal deletion" and is a major mechanism of the development of tolerance. It ensures that T-cells do not react with self (non-dangerous) antigens.
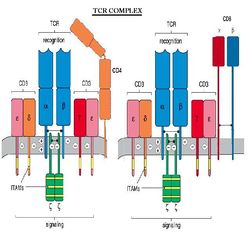
TCR Complex
The T cell Receptor, or TCR is always associated with CD3 which is referred to as the TCR complex. TCR is expressed on the surface of T cells in a noncovalent association with a complex of transmembrane polypeptides.
CD3 contains 3 distinct polypeptide chains that are expressed exclusively on T cells: γ, ε, and δ. These molecules are members of the Ig superfamily - the ε chain associates with both γ and δ - and they play a 'chaperone' role in transporting newly synthesized TCR molecules to the cell surface. CD3 also contains 2 identical chains: ζ and 16 kDa, which are found on T cells, macrophages and NK cells. Mice also can have an ε (eta) form.
Response to Activation
The response of the T cells to obtaining Signals 1 and 2 is to express the receptor for the cytokine interleukin-2 (IL-2) and CD4+ T-cells secrete IL-2. The final trigger for clonal expansion is the engagement of IL-2R with IL-2 from any activated CD4+ T-cell. IL-2 produced by a CD4+ cell may also stimulate clonal expansion of the CD4+ cell.
T-Helper Cell Function
The function of T helper cells is to regulate the immune response. The cytokines they secrete exert their influence on other cell populations; most of the different effector cells of the immune system are affected by one or more of the cytokines secreted by TH cells.
TH cells secrete cytokines for only a short period after they have been activated; the range of cytokines that TH cells secrete after activation chiefly determines their function. Different T-helper cell subpopulations (Th1 and Th2 cells) secrete different sets of cytokines.
Este artículo ha sido traducido de Inglés por 'Gregorio Puga Barlón' - 16.09.2011. |